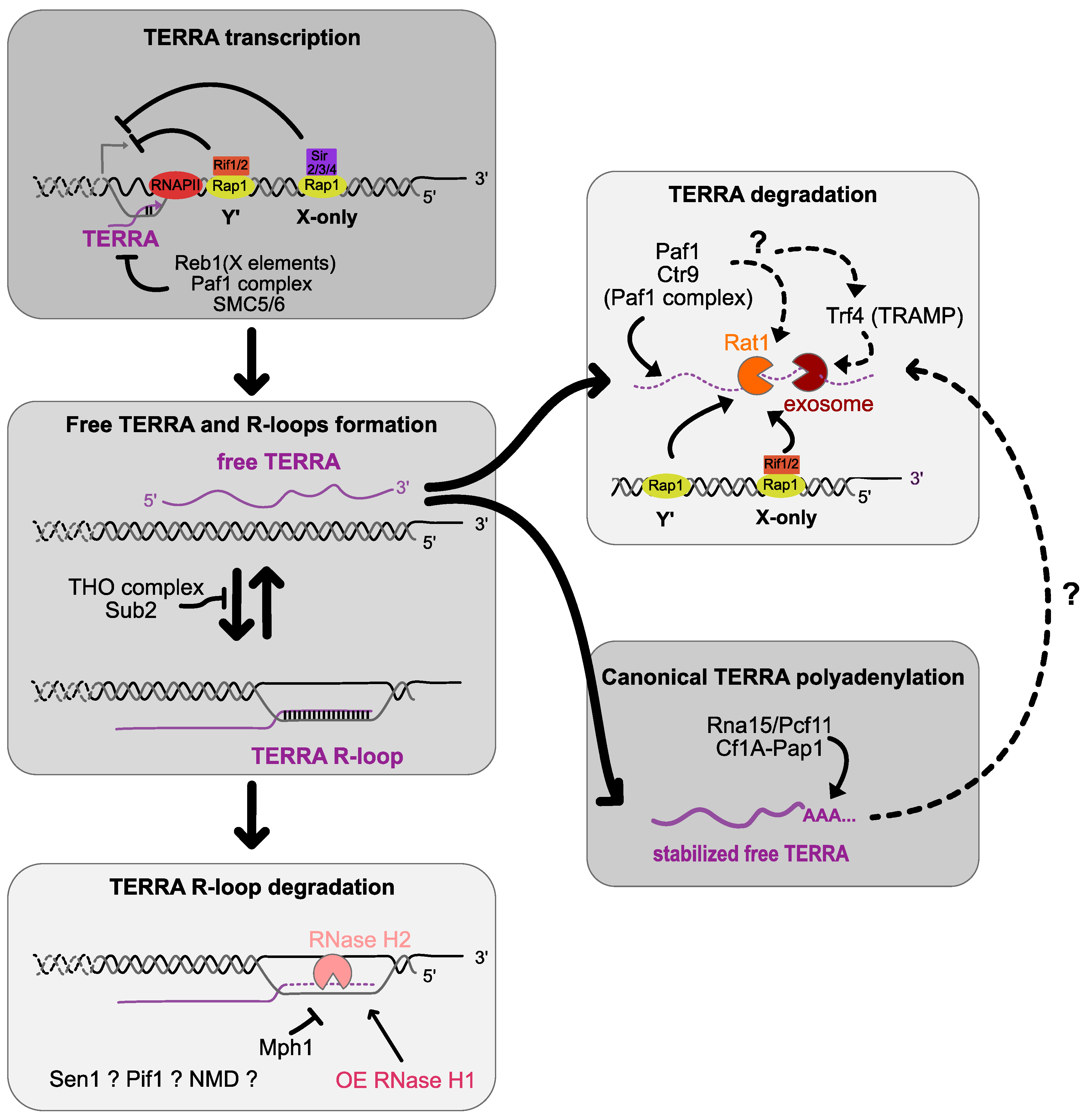
Activity of telomere maintenance mechanisms in healthy human tissues Biology Diagrams The basic N-terminal domain of TRF2 limits recombination endonuclease action at human telomeres. Cell Cycle 13, to DNA replication and repair and telomere maintenance. Mol. Biol. Cell 23, The telomere-shelterin complexes that cap all eukaryotic chromosomes ensure that healthy cells can progress through the cell cycle by preventing the cellular DNA damage response from identifying chromosome ends as double-stranded breaks (DSBs). [4] [5] Without a protective cap, chromosome ends would appear identical to intrachromosomal DSBs.These DSBs activate a DNA damage response pathway

Recently determined structures of the telomere maintenance protein complexes shelterin and CST shed new light on the regulation of telomere DNA replication and chromosome end-capping, and on how Telomeres are distinctive structures that protect the ends of linear chromosomes and ensure genome stability. They are composed of long tracks of repetitive and G-rich DNA that is bound by shelterin, a dedicated six-subunit protein complex. In somatic cells, shelterin protects telomeres from the DNA damage response and regulates telomere length. Telomere repeats are replenished by telomerase Telomere maintenance requires the telomerase and a network of telomere-associated proteins, (TIF), telomere end-end fusion, and ultimately cell cycle arrest, senescence, and genome instability [13, 14]. In human, telomere dysfunction has been implicated in bone marrow failure syndromes, leukemia, and cancer development [9, 10, 15

Telomere maintenance and the DNA damage response: a ... Biology Diagrams
Summary of mechanisms by which the DNA damage response is repressed at telomeres or harnessed to facilitate telomerase-mediated telomere maintenance. (A) Shelterin protects telomeres from inappropriately activating a DNA damage response in phases of the cell cycle when the telomere can fold into a t-loop. TRF2 promotes t-loop formation, which This event then stimulates DDR pathways, leading to increased expression levels of the cell cycle inhibitors p16 and p21, which in turn restrain proliferation [3,4]. Despite being shortened, these telomeres still maintain an adequate number of telomere-binding proteins to prevent fusion and block DNA repair [5-8]. This process fuels a However, repair at telomeres was only observed in proliferating cells, such as BJ fibroblasts and HeLa cells. Interestingly, HeLa cells, which are faster dividing cells, display a faster repair kinetics compared to slower dividing fibroblasts, suggesting that proliferation rate is an important determinant of telomeric DSB repair (Mao et al., 2016).